Chemical Reactions And Equations
| Taxtbook | NCERT * |
| Class | Class 10th |
| Subject | Science |
| Chapter | Chapter 01 |
| Topic | Chemical Reactions And Equations |
Chemical Reaction:
The process in which new substances with new properties are formed from one or more substances is called Chemical Reaction.
* The substances which take part in chemical reaction are called Reactants.
* The substances which are formed in a chemical reaction are called Products.
Examples:
(i) Digestion of food
(ii) Respiration
(iii) Rusting of iron
(iv) Burning of Magnesium ribbon
(v) Formation of curd
Chemical reaction involves :
● Change in state
● Change in colour
● Change in temperature
● Evolution of gas
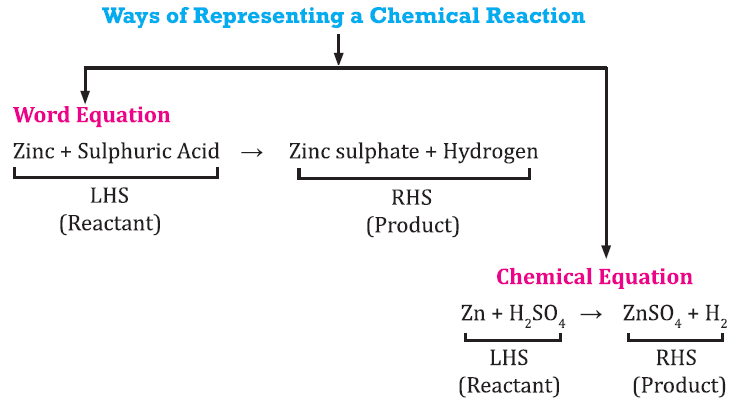
Chemical Equation
* A chemical reaction can be represented by chemical equation. It involves uses of symbol of elements or chemical formula of reactant and product with mention of physical state.
* The necessary conditions such as temperature, pressure or any catalyst should be written on arrow between reactant and products.
e.g., Magnesium is burnt in air to form magnesium oxide.
Mg + O2 → MgO
Balancing Chemical Equation
* Law of conservation of Mass Matter can neither be created nor destroyed in a chemical reaction.
* So number of elements involved in chemical reaction should remain same at reactant and product side.
STEPWISE BALANCING (Hit and Trial)
Step 1. Write a chemical equation and draw boxes around each formula.
Fe + H2 O → Fe3 O4 + H2
* Do not change anything inside the box.
Step 2. Count the number of atoms of each element on both the sides of chemical equation.
| Element | Number of atoms on reactants side (LHS) | Number of atoms on products side (RHS) |
| Fe | 1 | 3 |
| H | 2 | 2 |
| O | 1 | 4 |
Step 3. Equalise the number of atoms of element which has maximum number by putting in front of it.
Fe + 4H2 O → Fe3 O4 + H2
Step 4. Try to equalize all the atoms of elements on reactant and product side by adding coefficient in front of it.
3Fe + 4H2 O → Fe3 O4 + 4H2
* Now all the atoms of elements are equal on both sides.
Step 5. Write the physical states of reactants and products.
3Fe (s) + 4H2 O (g) → Fe3 O4 (s) + 4H2 (g)
Solid state = (s)
Liquid state = (l)
Gaseous state = (g)
Aqueous state = (aq)
Step 6. Write necessary conditions of temperature, pressure or catalyst on arrow above or below.
TYPES OF CHEMICAL REACTIONS
I. COMBINATION REACTION:
The reaction in which two or more reactant combine to form a single product.
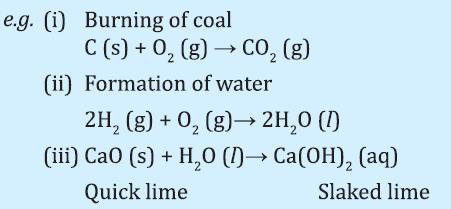
Exothermic Reactions:
Reaction in which heat is released along with formation of products.
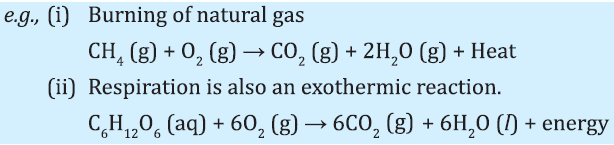
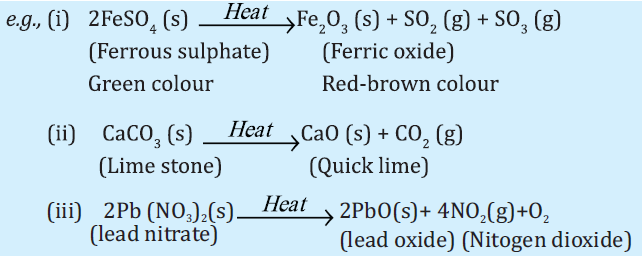
II. DECOMPOSITION REACTION:
The reaction in which a compound splits into two or more simple substances is called decomposition reaction.
e.g., A → B + C
Thermal decomposition:
When decomposition is carried out by heating.
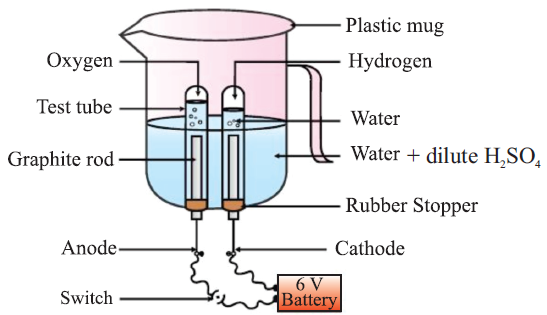
Electrolytic Decomposition: When decomposition is carried out by passing electricity.
Photolytic Decomposition: When decomposition is carried out in presence of sunlight.
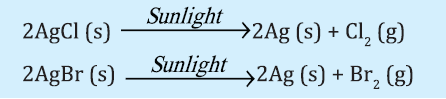
Above reaction is used in black & white photography.
Endothermic Reactions:
The reactions which require energy in the form of heat, light or electricity to break reactants are called endothermic reactions.
III. DISPLACEMENT REACTION:
The chemical reaction in which more reactive element displaces less reactive element from its salt solution.
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
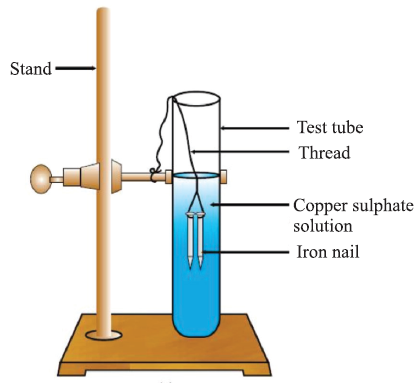
The iron nail becomes brownish in colour by deposition of Cu and blue colour of CuSO4 changes to dirty green colour due to formation of FeSO4.
Zn + CuSO4 → ZnSO4 + Cu
Zn is more reactive than copper.
IV. DOUBLE DISPLACEMENT REACTION:
A reaction in which new compounds are formed by mutual exchange of ions between two compounds.
(i) Na2 SO4 (aq) + BaCl2 (aq) → Ba SO4 (s) + 2NaCl (aq)
white precipitate of BaSO4 is formed, so it is also called precipitation reaction.
Note: All double displacement reactions are not precipitation reactions.
(ii) 2KI + Pb(NO3)2 → PbI2 + 2KNO3
(iii) 2KBr + BaI2 → 2KI + BaBr2
V. OXIDATION AND REDUCTION:
Oxidation:
(i) The addition of oxygen to reactant.
(ii) The removal of hydrogen from a reactant.
C + O2 → CO2
2Cu +O2 → 2CuO
2CuO + H2 → Cu + H2 O
Reduction:
(i) The addition of hydrogen to reactant.
(ii) The removal of oxygen from a reactant.
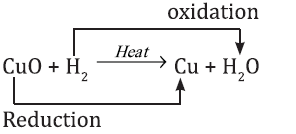
In this reaction CuO is reduced to Cu and H2 is oxidized to H2O. So, oxidation and reduction taking place together is redox reaction.
Effects of Oxidation in Daily Life
(1) Corrosion :
● When a metal is exposed to moisture, air, acid etc. for some time, a layer of hydrated oxide is formed which weakens the metal and hence metal is said to be corroded.
● Rusting of iron, black coating on silver and green coating on copper are examples of corrosion.
● Corrosion can be prevented by galvanization, electroplating or by putting paints.
(2) Rancidity: The oxidation of fats and oils when exposed to air is known as rancidity. It leads to bad smell and bad taste of food.
Methods to Prevent Rancidity
(i) By adding antioxidants
(ii) Keeping food in air tight containers
(iii) Replacing air by nitrogen
(iv) Refrigeration
